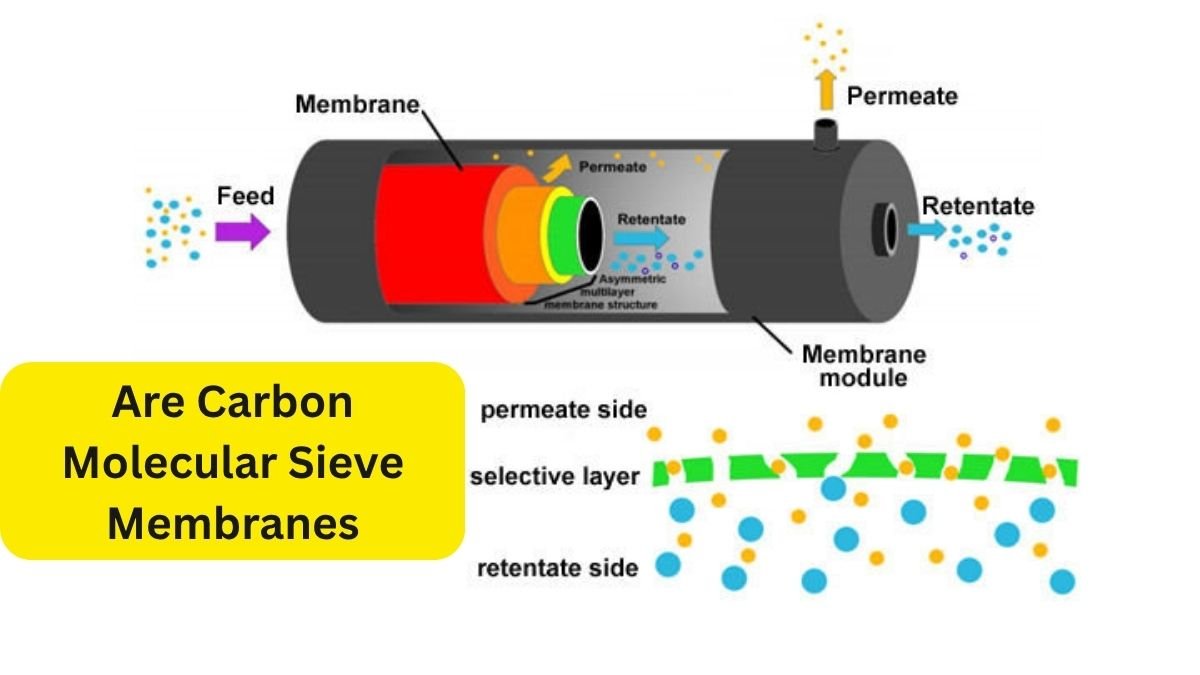
Chemical separation processes are essential in many industries, especially in the production of fuels, chemicals, and materials. However, these processes are also some of the most energy-intensive operations worldwide. In fact, nearly half of all industrial energy use goes toward chemical separations, with distillation alone accounting for about 10% to 15% of global energy consumption.
Traditional separation methods like distillation and adsorption are widely used but are often energy-hungry and expensive to maintain. This is where membrane-based separation technologies come into the picture. Compared to conventional methods, membrane separations use much less energy and cost less to maintain, making them a more sustainable choice in many applications.
Most membranes currently used in industry are made of polymers.
These polymeric membranes are popular because they’re relatively easy to manufacture and can be shaped into different forms to suit various applications. But there are also some downsides. Polymeric membranes tend to degrade when exposed to high temperatures, making them unsuitable for certain industrial processes. They also suffer from a phenomenon called plasticization, which reduces their ability to separate gases effectively.
When we talk about the performance of a membrane, two main factors come into play: permeability and selectivity. Permeability refers to how easily a gas can pass through the membrane, while selectivity indicates how well the membrane can distinguish between different gases. Unfortunately, there’s usually a trade-off between these two. If a membrane has high permeability, it tends to have low selectivity, and vice versa. This trade-off is known as Robeson’s upper bound—a benchmark that represents the performance limit for polymeric membranes in gas separations.
To overcome this limitation,
researchers have been exploring advanced materials like carbon molecular sieve membranes (CMSMs). These inorganic membranes have the potential to surpass the Robeson limit, offering both high permeability and high selectivity. CMSMs are attracting increasing attention because they could be the key to making industrial separations much more efficient and sustainable.
Meanwhile, scientists are also working on improving the overall efficiency of chemical production by developing new catalysts, processes, and adsorbents. A promising innovation in this area is the membrane reactor. According to the International Union of Pure and Applied Chemistry (IUPAC), a membrane reactor is a device that combines a chemical reaction and a separation process in one unit. For example, in steam reforming or dry reforming, the membrane not only helps with separating products but can also shift the reaction equilibrium to improve yield and selectivity. This integration leads to better performance than traditional systems.
Carbon molecular sieve membranes are seen as ideal candidates for membrane reactors and gas separation. Here’s why they are promising:
- They are relatively easy to manufacture.
- The raw materials and production costs are low.
- Their structure allows for molecular sieving, enabling precise separations.
- They offer high gas permeance.
- They can withstand conditions that would damage polymeric or palladium membranes.
The concept of CMSMs dates back to 1983 when Koresh and Sofer from Israel’s Atomic Energy Commission first developed carbon membranes by carbonizing thermosetting polymers. These membranes had pores smaller than gas molecules, which led to significantly better gas separation performance compared to polymeric membranes.
More recently, researchers like Salleh and Ismail have reviewed and documented the latest progress in CMSM technology, highlighting some of the most promising membranes currently available. These include both lab-developed and commercially available membranes, with particular focus on hydrogen production.
CMSMs are made from materials rich in carbon and have a complex pore structure composed of micropores and ultramicropores. These tiny pores allow for precise control over which gas molecules can pass through. The membranes have excellent thermal stability and chemical resistance, making them suitable for use in harsh industrial environments.
Their structure is made up of imperfectly.
stacked layers of graphene, referred to as a turbostratic structure. This creates nanoscale slit-like openings that are responsible for the separation. The micropores allow fast gas transport, while the ultramicropores contribute to the molecular sieving ability. Unlike crystalline materials, CMSMs are amorphous, meaning their pore sizes vary.
These membranes separate gases using two main mechanisms: molecular sieving and selective surface diffusion. In molecular sieving, gases are separated based on size. Only smaller molecules can fit through the tiny pores, while larger ones are blocked. For this to work effectively, it’s crucial to have precise control over the pore size.
In selective surface diffusion,
the membrane favors gases that are more adsorbable. These gases stick to the surface of the membrane, move across it, and then exit on the other side. This allows for the separation of strongly adsorbable gases like carbon dioxide (CO2) and ammonia (NH3) from weakly adsorbable gases like nitrogen (N2) and methane (CH4).
CMSMs are made by heating polymeric precursors in an inert or vacuum environment to temperatures between 500°C and 1000°C. This process, known as carbonization, removes volatile elements from the polymer, leaving behind a porous carbon structure. The final properties of the membrane depend on several factors, including the type of polymer used and the carbonization conditions.
Common polymers used include polyimides, polyfurfuryl alcohols, phenolic resins, and polyacrylonitrile. These materials must be thermosetting, meaning they don’t melt before breaking down. Carbonization parameters like heating rate, temperature, and atmosphere must be carefully controlled. Post-treatments like oxidation or activation can also fine-tune the membrane’s pore structure.
For instance, heating the membrane in oxygen between 100°C and 450°C—a process known as oxygen doping—can adjust the size and distribution of pores. This helps in separating gases with very similar sizes, such as ethylene and ethane.
Other researchers have focused on the casting process and drying techniques to improve performance. Zhang and colleagues found that using high-boiling-point solvents and cold-drying methods led to membranes with better thermal stability and gas separation efficiency. They achieved impressive oxygen permeabilities and selectivities, depending on the carbonization temperature.
Similarly, new types of polymer membranes, known as polymers of intrinsic microporosity (PIMs), have shown promise. These materials have very high surface areas and extremely small pores, making them excellent candidates for carbonization into CMSMs. One such membrane achieved record-setting performance for separating ethylene from ethane.
To further enhance performance,
researchers are also developing composite CMSMs (c-CMSMs). These are membranes that incorporate inorganic materials like metals or zeolites into the carbon structure. The added materials can increase pore volume and improve gas separation. For example, adding silver nanoparticles can create selective pathways for gases or enhance the membrane’s structure.
Other innovations include combining CMSMs with alumina or zeolite materials. These hybrid structures have achieved gas separation performance that exceeds the Robeson upper bound. Nickel-doped CMSMs and hydrophobic carbon-silica membranes have also shown great promise in applications like hydrogen purification and oxygen/nitrogen separation in humid conditions.
In summary, carbon molecular sieve membranes and their composites are at the forefront of next-generation gas separation technologies. They offer the potential to drastically reduce energy consumption in chemical processes, making them a key component in building a more sustainable industrial future.