The world of chemistry of living beings operates primarily in solutions dissolved in water. Keeping the concentration of these solutions balanced inside and outside the cells is a constant challenge. The main protagonist of this balance is the cell membrane, which controls the movement of substances.
The cell membrane is not just a simple layer but acts like a smart ‘security guard’ that decides which substance comes in and which goes out. Let’s understand this step by step.
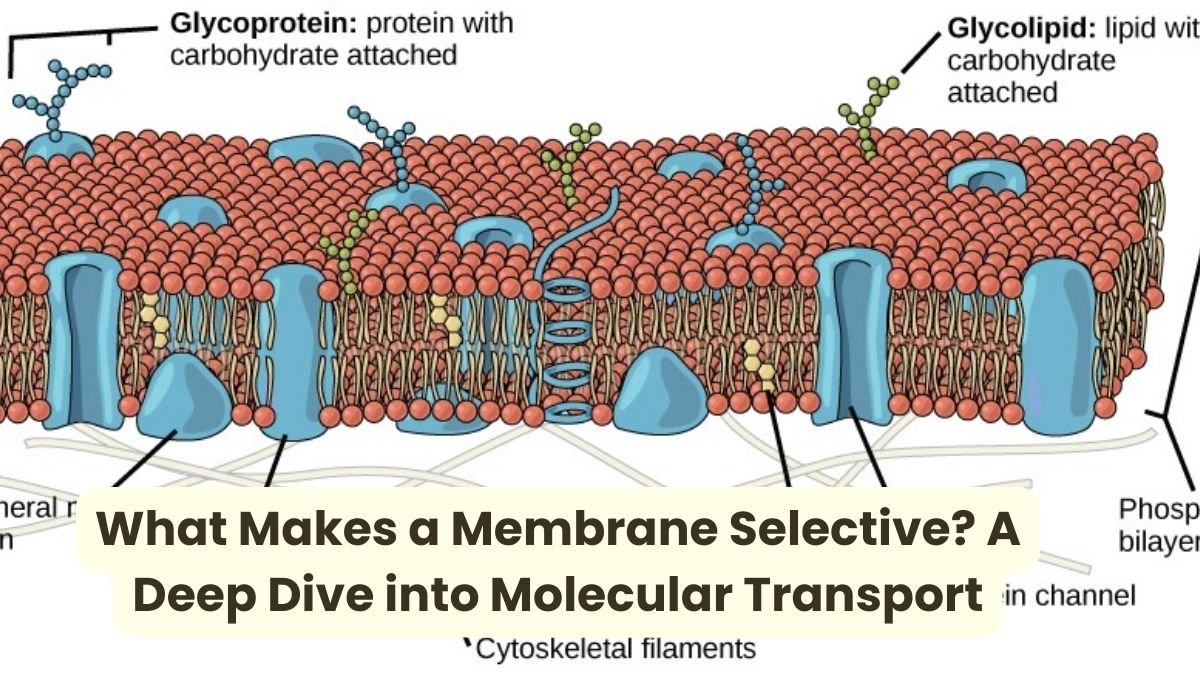
Function and Challenge of Cell Membrane
The chemical substances that one may find inside the cell include ions (Ca 2+, Na 1+, K1+, Cl 1-) nutrients (sugars, fatty acids, amino acids) and wastes (such as CO 2). The membrane has to be informed about what to take in, what to expect and when.
Design Challenge:
Letting the right substance in and out at the right time.
Membrane structure and selectivity
The cell membrane is made up of a lipid bilayer. Its middle part is hydrophobic (water fearing), due to which it allows only certain substances to pass through.
Selective permeability means that only certain substances can cross the membrane without help.
Small and nonpolar molecules enter easily.
Large or charged molecules require protein channels or transporters.
Relative permeability and MPC
Different substances cross the membrane at different speeds. Membrane Permeability Coefficient (MPC) is used to measure this speed.
- Short MPC = slow and rapid transits (e.g. hexanoic acid, MPC = 0.9 cm/s)
- Moderate MPC = mean velocity ( e.g. water, ethanol, MPC = 0.01-0.001 cm/s)
- Very low MPC = very slow ( e.g sodium ions, MPC = 10-12 cm/s )
However, this rate is influenced by a lot of factors distribution of membranes, temperature, size, charges, solubility, and other physical properties of chemicals.
Kinds of transport
The two key ways by which substances are allowed to pass through membranes are:
Passive transport none (energy wise) is utilized, substances comes in high concentration to low concentration.
Active transport – Against the concentration gradient forces are moved by energy utilization (ATP)
Passive Transport
4.1 Diffusion
In diffusion, molecules diffuse so they end up getting cavities in an area with low concentration as the concentration stays the same.
Example: breaking a bottle of ammonia in the room — the odour diffuses gradually in the room.
Factors affecting the speed of diffusion:
- Difference in concentration gradient
- Size and mass of the molecule
- Temperature
- Density of solvent
- Solubility of the molecule
- Surface area and thickness of the membrane
- Distance (greater distance = slower diffusion)
4.2 Facilitated Diffusion
There are chemical products, which are unable to traverse the lipid bilayer directly; examples of these are ions and polar molecules. The transport proteins or channel proteins facilitate these.
Channel protein: a ‘door’ that allows specific molecules to pass through.
Aquaporin: special channels for water molecules that allow water to pass through the membrane quickly.
Facilitated diffusion also does not expend energy, but it is not possible without proteins.
Scientific reasons behind membrane selectivity
Several factors work in determining the permeability of the membrane:
- Size of the molecule – Small molecules pass through faster.
- Polarity – Non-polar molecules penetrate easily.
- Charge – Protein channels are required for charged particles.
- Temperature and environment – Movement is faster at higher temperatures.
- Composition of membrane – Different types of membranes have different permeability.
Importance in biology
If the membrane is not selective, the internal environment of the cell will deteriorate, and life will become impossible.
Example:
- If Na⁺ ions enter unchecked, nerve impulses may be disrupted.
- If there is an imbalance of water, the cell may swell or burst.
- Therefore, selective permeability is a fundamental pillar of life.
Conclusion
The cell membrane is an amazing, subtle and intelligent structure, which controls the movement of substances based on chemical and physical rules.